Diastereoselective Synthesis of 1,2-Dihydrobenzofuro[3,2-b]pyridines via a Carbon-Carbon Double Bond Cleavage–Rearrangement Cascade
Chi-Fan Zhu, Ling-Qi Chen, Wen-Juan Hao,* Chen-Chang Cui, Shu-Jiang Tu,* Bo Jiang*
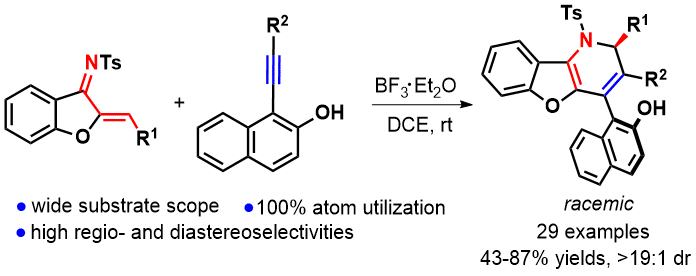
A new Lewis acid-catalyzed [2 + 2] cycloaddition-retroelectrocyclization (CA-RE)/1,6-addition relay of aurone-derived 1-azadienes and 1-alkynylnaphthalen-2-ols has been reported, leading to regio- and diastereoselective synthesis of 1,2-dihydrobenzofuro[3,2-b]pyridines with a chiral carbon center and an axial chirality in good yields. This protocol enables the C–C double bond scission/recombination to rapidly construct aza-heterocyclic architectures and features 100% atom utilization, wide substrate scope and good compatibility with substituents as well as excellent diastereoselectivity.
Org. Lett. 2021, 23, 2654-2658; DOI: 10.1021/acs.orglett.1c00564